McKinsey to pay $650 mn to settle US criminal case on opioids

Source: AFP
PAY ATTENTION: NOW You can COMMENT on our articles on the YEN website! Learn how to get started.
Consulting giant McKinsey & Company will pay $650 million to settle US criminal charges that it collaborated with Purdue Pharma to intentionally misbrand opioids, worsening a public health crisis, officials announced Friday.
US prosecutors unveiled a sweeping deferred prosecution agreement (DPA) over McKinsey efforts that helped Purdue "turbocharge" sales of the highly addictive OxyContin opioid, responsible for deadly overdoses.
The accord, which requires McKinsey to implement a comprehensive compliance program, represents the "first time a management consulting firm has been held criminally responsible for advice that it has given resulting in the commission of a crime by a client," said US Attorney Christopher Kavanaugh, of the Western District of Virginia, speaking at a news conference.
Additionally, prosecutors charged McKinsey US with one felony count of knowingly destroying evidence with an intent to impede a probe and a misdemeanor of knowingly conspiring with Purdue to abet the misbranding of drugs, the Justice Department said.
The DPA, which will expire in five years if McKinsey meets the conditions, also noted that a senior partner with the prominent consultancy "knowingly" destroyed and hid records with the "intent to impede, obstruct and influence" the probe.
Prosecutors said the former McKinsey partner, Martin Elling, had agreed to plead guilty in the case.
From 1999 through 2022 nearly 727,000 people died from opioid overdoses, according to the Centers for Disease Control and Prevention.
McKinsey expressed regret for the firm's role in the scandal.
"We are deeply sorry for our past client service to Purdue Pharma and the actions of a former partner who deleted documents related to his work for that client," McKinsey said in a statement. "We should have appreciated the harm opioids were causing in our society and we should not have undertaken sales and marketing work for Purdue Pharma."
McKinsey has bolstered its risk management practices, it said, adding, "though we wish we had taken these steps sooner, we are committed to building on them to ensure that McKinsey sets the standard for accountability and compliance across our profession."
Friday's announcement comes about a week after US prosecutors in New York unveiled a nearly $123 million settlement with McKinsey and Co. Africa in a separate DPA, over charges the subsidiary paid bribes to South African officials to obtain lucrative consulting contracts.
'High- Value Prescribers'
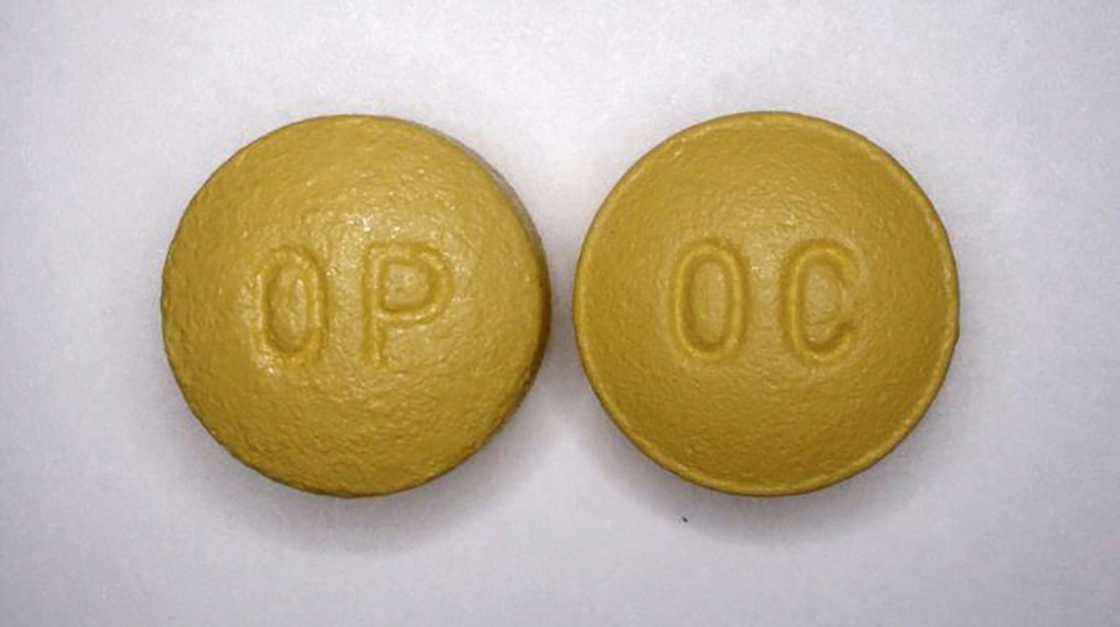
Kavanaugh described McKinsey as intimately involved in efforts to boost OxyContin sales after they fell off following a 2007 US settlement with Purdue over criminally misbranding the drug.
Undertaking a study for client Purdue, McKinsey determined that the sales dropped due to concerns by doctors and pharmacists that the drug was being abused.
"To turbocharge sales, McKinsey devised, and Purdue followed, the targeting of High Value Prescribers, including those who are prescribing opioids for uses that were unsafe, ineffective and medically unnecessary," Kavanaugh said.
Source: AFP
"This was not just marketing," he added. "It was strategy. It was executed and it worked."
The settlement requires McKinsey to establish and provide updates on a compliance program that involves employee training and risk-assessment reviews of potential clients. The company must also undertake new document retention policies.
Such measures constitute "a full infrastructure of what professional services firms are expected to do to make sure they don't end up in this situation," said US Attorney Joshua Levy.
McKinsey also agreed not to do any work "related to the marketing, sale, promotion or distribution of controlled substances," the filing said.
Friday's criminal agreement comes on the heels of earlier civil McKinsey settlements over its conduct relating to Purdue.
In February 2021, McKinsey agreed to pay $573 million to settle claims brought by 47 states and five US territories that it contributed to the opioid crisis through its advice to pharmaceutical giants.
In September 2023, McKinsey agreed to an additional $230 million in settlements with US municipalities, counties and public school districts.
New feature: Сheck out news that is picked for YOU ➡️ click on “Recommended for you” and enjoy!
Source: AFP




