FDA Warns Public About Contaminated Eyedrop Brands After 8 People Go Blind, 3 Dead
- The FDA has warned the Ghanaian public about a contaminated eyedrop product that could find its way into the country
- The FDA has disclosed in a statement dated March 23, 2023, that Ezricare Artificial Tears and Delsam Pharma’s Artificial Tears have caused blindness in 8 people and killed 3 in the US
- FDA said while it has authorised the products to be sold on the Ghanaian market, Ghanaians should remain vigilant and stay away from using them
PAY ATTENTION: Enjoy reading our stories? Join YEN.com.gh's Telegram channel for more!
Ghana's Food and Dr@gs Authority (FDA) has issued an emergency alert over two foreign eyedrop brands wreaking havoc in the United States.
The FDA has said in a statement issued on Thursday, March 23, 2023, that the two brands of eyedrops, Ezricare Artificial Tears and Delsam Pharma’s Artificial Tears.
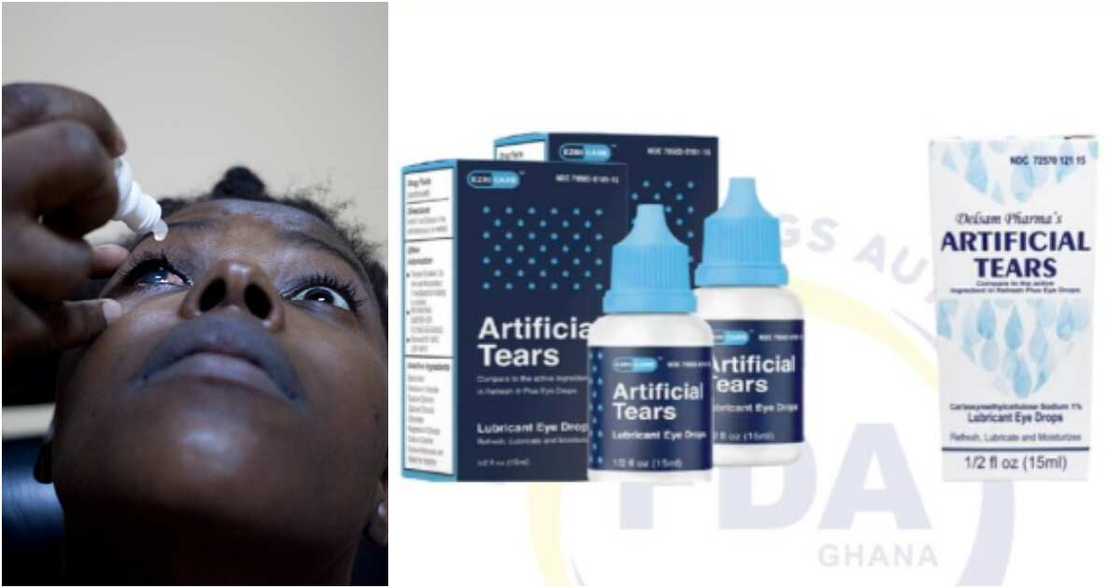
Source: UGC
The Authority disclosed that the two eyedrop brands have been linked to the death of three people and vision loss in eight others.
Already the US Centre for Disease Control and Prevention has classified the incident as an outbreak and the manufacturer of the two types of eyedrops, Global Pharma, has already recalled the products from the US markets.
PAY ATTENTION: Follow us on Instagram - get the most important news directly in your favourite app!
"The products are not registered with Ghana FDA. Therefore, they should not be available on the Ghanaian market. However, the FDA advises the public who may be in possession of these dr@gs through other means to immediately stop using the recalled products, Ezricare Artificial Tears and Delsam Pharma's Artificial Tears, and submit them to any of the FDA offices nationwide," the FDA said in the statement.
The FDA wants anyone who has used the products that have been recalled and is experiencing any adverse effects to quickly reach out to a health professional.

Source: Facebook
FDA acts fast as Yellow Sisi Waakye at Oyibi kills one person
Meanwhile, YEN.com.gh reported in a previous story that the FDA suspended the operations of a food business after cases of food poisoning were reported.
The suspension comes after some 53 persons were affected and one person died after consuming either waakye or plain rice and tomato stew from a food vendor called Yellow Sisi.
In a statement issued by the FDA, it said after assessing the venue and the food served, they realised that unhygienic practices and poor handling of food took place at the food joint.
In that report, some Ghanaians shared their thoughts on the action of the FDA in suspending the food business.
Marwako Fast Food laments GH¢750,000 FDA fine over food poisoning incident
Also, Marwako Fast Food has revealed that the FDA slapped a fine of GH¢750,000 on them following the food poisoning incidents.
The fast food chain said while it is determined to comply with all FDA regulations to resolve the issue, it is important for the regulator to treat them fairly.
The FDA has accused Marwako of single-handedly destroying food items while investigations into the food poisoning were ongoing.
New feature: Сheck out news that is picked for YOU ➡️ click on “Recommended for you” and enjoy!
Source: YEN.com.gh




